mRNA Therapeutics
Why mRNA represents a disruptive new drug class
In the last decade mRNA has progressed into a promising new class of medicine, with the potential to treat a wide variety of diseases with high unmet medical needs. mRNA is a long, polymeric molecule, composed of four different building blocks called nucleotides. In mRNA, hundreds or thousands of these nucleotides are linked in a unique order to convey genetic information to cells, where it is used to express proteins with biological effects.
Considering that all mRNA is generated with four different building blocks, but with unique sequence order, all therapeutic mRNAs have highly similar compositions, while having the capacity to encode a variety of different proteins. These characteristics allow for rapid development of mRNA therapeutics that are broadly applicable for treatment of many diseases, including cancer, infectious diseases and rare diseases. Our mRNA pipeline addresses all of these therapeutic areas.
Our mRNA technologies
The structural elements of the mRNA have an impact on its performance. This includes potential immunogenicity, efficacy of translation and stability of the molecule. We leverage our extensive experience to design, synthesize, manufacture and formulate our therapeutic mRNA, and adapt its composition to suit the desired application.
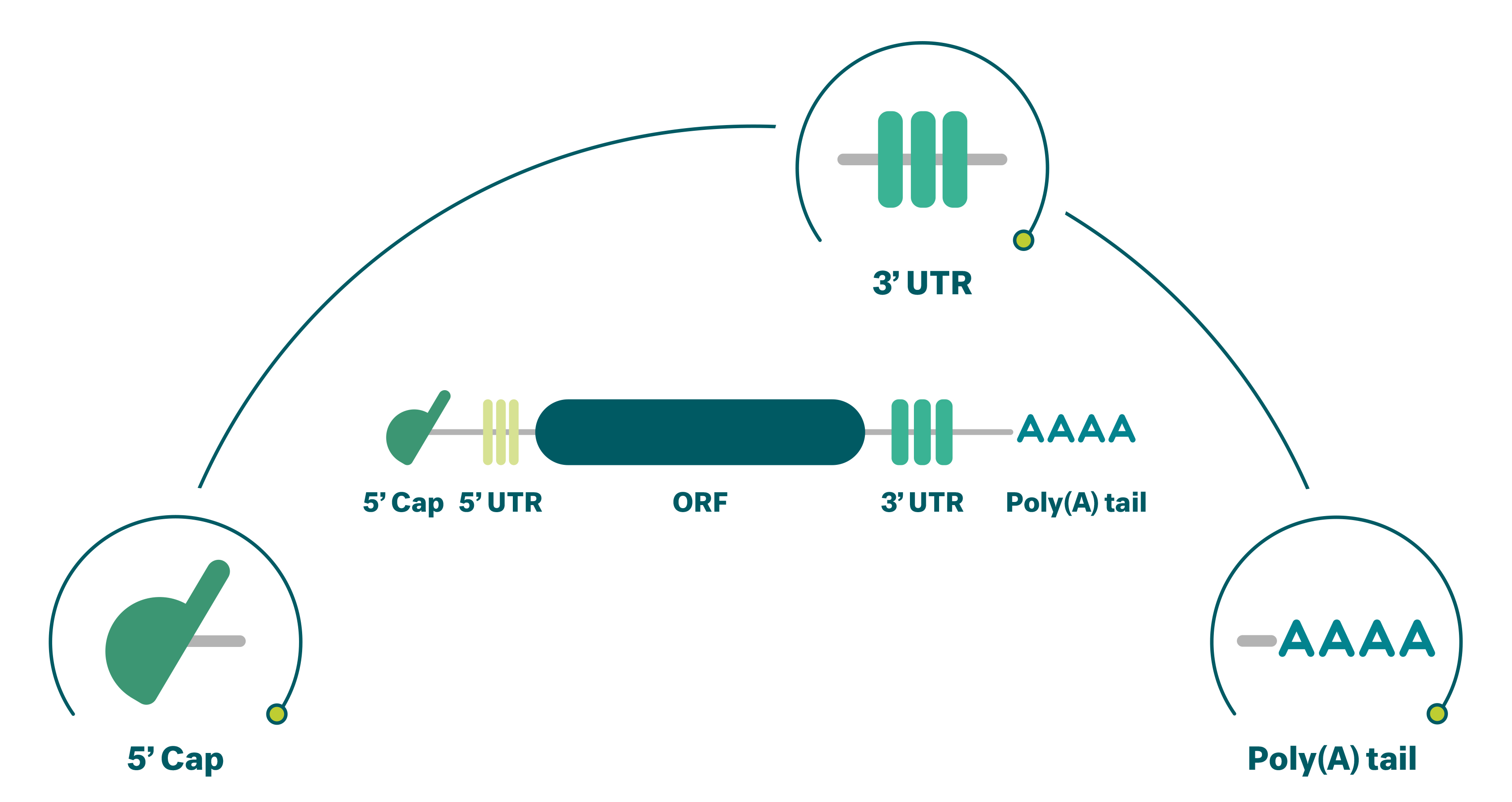
Our mRNAs all contain basic structural elements that we believe are critical for successful development:
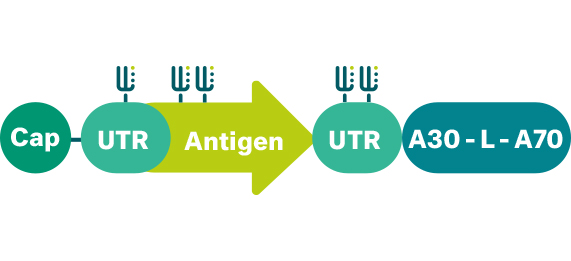
- 5’ cap: Incorporation of a unique cap analogue into the mRNA helps to achieve superior translational performance by stabilizing the mRNA molecule and directing the immune response.
- 3’ untranslated region: The composition and structure of the 3’ untranslated regions of the mRNA molecule are important determinants of the intracellular stability of mRNA.
- Poly(A) tail: We have performed extensive research on the structure of the poly(A) tail and the translational performance of mRNA and customized our template design accordingly.
Our mRNA formats include:
The nucleotide sequence of mRNA determines the amino acid sequence of the protein. In addition, the nature of nucleosides used for production of mRNA drugs can also influence recognition of the molecule by the immune system. Presence of naturally occurring uridine (U) in our optimized mRNA makes it immunogenic by activating immune sensors. We have further optimized our unmodified mRNA for immunogenicity (augmented antigen presentation on MHC I and MHC II) and pharmacological activity (enhanced stability and translational efficiency). Immunogenicity of the mRNA is an added benefit when mRNA is used for immunotherapy applications, by acting as an immunotherapy adjuvant. This makes our therapeutics for iNeST and FixVac even more potent.

Repeat administration
Strong T cell responses
Immunogenic reaction against mRNA drugs needs to be avoided in applications where therapeutic proteins are produced, such as in our RiboMab and RiboCytokine platforms. We have profound expertise in incorporating naturally-occurring modified nucleosides into our therapeutic mRNAs. We have demonstrated that the presence of a variety of modified nucleosides in the manufactured mRNA suppresses its intrinsic immune activation, while leading to superior protein production for long duration. Deimmunizing mRNA by incorporating modified nucleosides helps to avoid production of anti-drug antibodies and broadens the therapeutic application of these types of mRNA drugs.
Non-immunogenic vector
Strong antibody responses
Therapeutic protein delivery
Our self-amplifying mRNA (saRNA) drugs use the concept of viral replication, while not being an infectious, disease-causing agent itself. saRNA resembles conventional mRNA encoding the protein of interest, but also encoding a polymerase, called replicase, that multiplies part of the mRNA within the target cell. During self-amplification inside the cell, a double-stranded RNA intermediate is generated, which is recognized by intracellular immune sensors. This makes saRNA a very potent activator of the immune system and therefore an excellent category of immunotherapy. As we have demonstrated, our saRNA ensures high levels of sustained antigen production with a small amount of initial mRNA input, which makes this mRNA class a potent tool for prophylactic vaccination, with the potential application in infectious diseases with high medical needs.
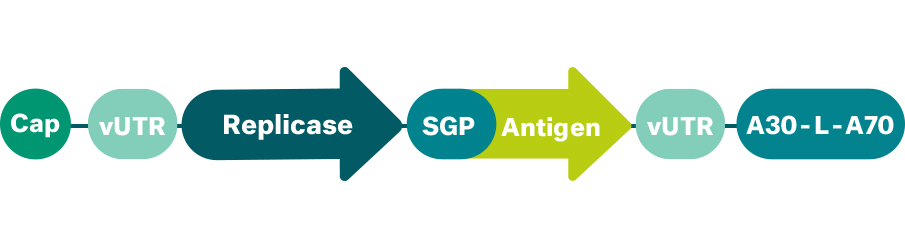
Sustained expression
T cell responses upon prime only
Protection upon prime only
This technology is an advancement of the saRNA platform. By separating the target mRNA to be amplified and the replicase encoding mRNA we have broadened the spectrum of applications. This makes the development of therapeutic mRNAs even more flexible, as the replicase can amplify mRNA encoding of not only one protein, but several different ones. In the case of vaccines, this allows us to produce the replicase in advance for use with different vaccines. Our trans-amplifying mRNA is a proprietary mRNA format that is particularly well-suited for prophylactic vaccines to prevent infectious diseases.
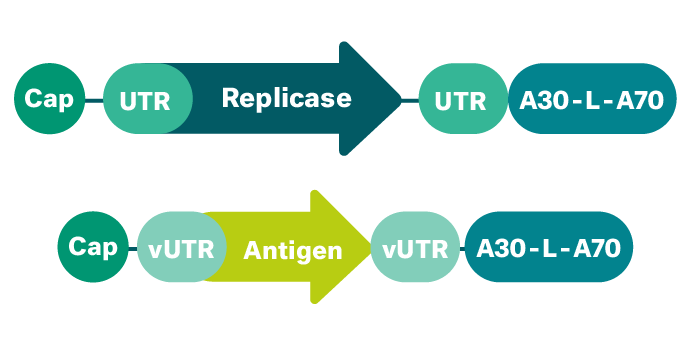
Sustained expression
Highly flexible co-transfer
Low antigen RNA doses
At a glance: mRNA as a Therapeutic Drug Class

- Natural molecule found universally within cells, with well-characterized properties.
- Suitable to encode for antibodies, antigens, cytokines and any other type of protein.
- Transient, with adaptable activity and half-life. Avoids genomic integration problems sometimes seen in gene therapy, potentially resulting in a better safety profile.
- Can be designed and optimized pharmacologically and immunologically, making it suitable for a broad range of applications.
- Fast manufacturability, making it an inexpensive and flexible therapeutic to produce.
Our mRNA Delivery Formulations
Our deep and broad expertise in the targeted delivery of mRNA therapeutics is a key strength of BioNTech
A mRNA drug needs to be appropriately formulated in order to be protected from degradation by extracellular RNAses. The right formulation is critical to ensure the appropriate delivery of the RNA to the intended site of action.
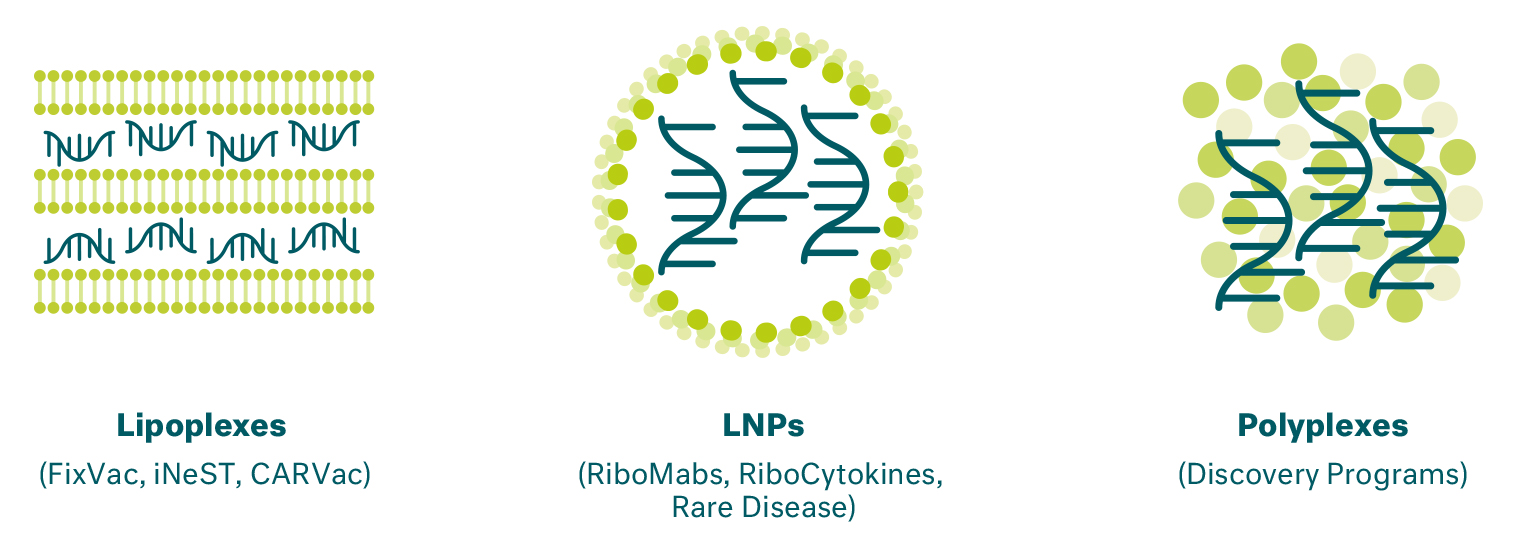
We employ multiple mRNA delivery formulations, each designed for different functions and optimized for therapeutic product needs based on the intended application and route of delivery:
- Lipoplex: Our lipoplex formulation, or LPX, embeds the mRNA between a lipid bilayer, which is used for our FixVac and iNeST platforms. We use a proprietary size- and charge-based non-viral mRNA lipoplex that was developed to deliver mRNA to dendritic cells in lymphoid compartments such as the spleen for optimal antigen presentation and immune response activation.
- Lipid Nanoparticles (LNPs): For other applications, we encapsulate our mRNA in lipid nanoparticles, or LNPs. These formulations are suitable for our RiboMab, RiboCytokine and rare disease protein replacement platforms. Our LNP formulations can be adjusted according to our needs for delivery to particular target tissues, such as the liver in the case of our rare disease protein replacement platform.
- Polyplexes: Our portfolio also comprises polyplexes, which are being utilized in certain of our discovery programs, in which the mRNA is bound to a polymer and then forms nanoparticles.
_0_0.png)